Isotope isotopes hydrogen isotopi wasserstoffs idrogeno isotopen worldatlas neutrons waterstof drie hydrog ne elettrone kol atoms diagramma tritium vecteurs
Table of Contents
Table of Contents
Are you struggling with how to draw isotopes in your Chemistry class? You’re not alone. Many students find it challenging to understand the concept of isotopes and how to draw them. In this article, we’ll discuss step-by-step instructions on how to draw isotopes, as well as tips and tricks to help you master this important part of Chemistry.
When it comes to how to draw isotopes, there are several pain points that many students face. Some of these include understanding the differences between isotopes, isobars, and isotones, as well as knowing how to identify the number of neutrons in an atom. Additionally, some students may struggle with balancing the number of protons and neutrons in a given isotope.
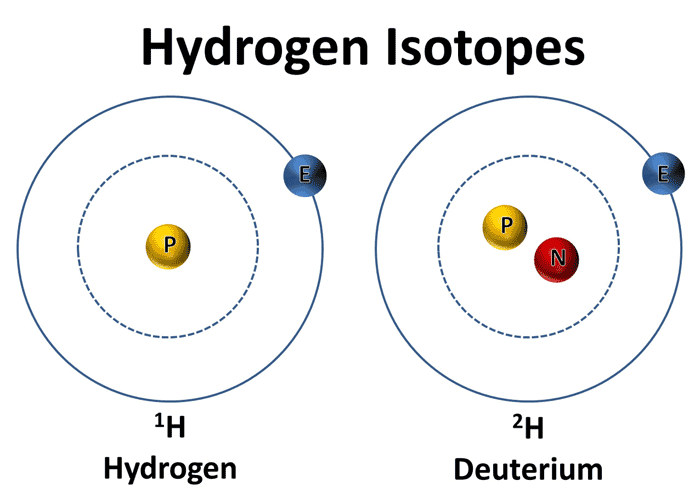
So, what is an isotope? Isotopes are atoms of the same element that have different numbers of neutrons. This means that while the number of protons (which determines the element’s atomic number) remains the same, the mass number (which is the sum of the protons and neutrons) differs. Drawing isotopes involves representing the nucleus of the atom, including the number of protons and neutrons.
In summary, how to draw isotopes involves understanding the differences between isotopes, isobars, and isotones, knowing how to identify the number of neutrons in an atom, and being able to balance the number of protons and neutrons in a given isotope. In the following sections, we’ll explore these concepts in more detail with step-by-step instructions and helpful tips.
How to Draw Isotopes: Understanding the Basics
When drawing isotopes, it’s important to first understand the structure of an atom. At the center of the atom is the nucleus, which is made up of protons and neutrons. Electrons orbit the nucleus in shells or energy levels.
To draw an isotope, we need to consider the number of protons and neutrons in the nucleus. The number of protons determines the element, while the number of neutrons can vary between isotopes. To represent an isotope, we can use its atomic symbol, which shows the element’s symbol, atomic mass, and atomic number.
 Let’s take a specific example to illustrate this concept. Consider the element carbon, which has an atomic number of 6. This means it has 6 protons in the nucleus. However, carbon can exist in several isotopes, depending on the number of neutrons in the nucleus. Carbon-12 has 6 protons and 6 neutrons (for a total atomic mass of 12), while carbon-13 has 6 protons and 7 neutrons (for a total atomic mass of 13).
Let’s take a specific example to illustrate this concept. Consider the element carbon, which has an atomic number of 6. This means it has 6 protons in the nucleus. However, carbon can exist in several isotopes, depending on the number of neutrons in the nucleus. Carbon-12 has 6 protons and 6 neutrons (for a total atomic mass of 12), while carbon-13 has 6 protons and 7 neutrons (for a total atomic mass of 13).
Tips and Tricks for Drawing Isotopes
Now that we know the basics of how to draw isotopes, let’s explore some tips and tricks to make the process easier.
First, it’s important to remember that isotopes of the same element have the same number of protons. Therefore, when drawing isotopes, we only need to change the number of neutrons in the nucleus.
Another helpful tip is to use the periodic table to determine the number of protons and electrons in an element. The number of protons is the same as the element’s atomic number, while the number of electrons is the same as the atomic number for neutral atoms.
 ### How to Draw Isotopes with Practice Problems
### How to Draw Isotopes with Practice Problems
Practice makes perfect when it comes to drawing isotopes. Here are some practice problems to help you master this skill.
1. Draw the isotope nitrogen-14.
Answer: Nitrogen has an atomic number of 7, so it has 7 protons in the nucleus. Nitrogen-14 has 7 protons and 7 neutrons (for a total atomic mass of 14).
2. Draw the isotope carbon-14.
Answer: Carbon has an atomic number of 6, so it has 6 protons in the nucleus. Carbon-14 has 6 protons and 8 neutrons (for a total atomic mass of 14).
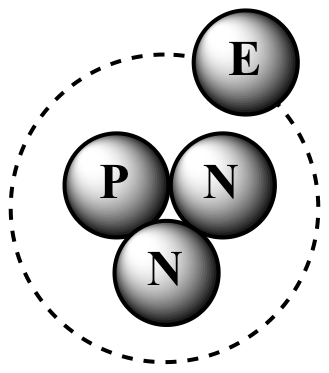 #### Common Mistakes When Drawing Isotopes
#### Common Mistakes When Drawing Isotopes
When learning how to draw isotopes, there are some common mistakes to avoid. One is mistaking isotopes for ions, which are atoms that have gained or lost electrons. Isotopes, on the other hand, have the same number of electrons as the element’s neutral state. Another mistake is mixing up the atomic number and atomic mass, which are two different concepts.
Conclusion of How to Draw Isotopes
Drawing isotopes is an important skill to master in Chemistry. By understanding the differences between isotopes, isobars, and isotones, as well as knowing how to identify the number of neutrons in an atom, you can successfully draw isotopes for any element. Remember to practice and use helpful tips to ensure accuracy and avoid common mistakes.
Question and Answer
Q: What is an isotope?
A: An isotope is an atom of the same element that has a different number of neutrons in the nucleus.
Q: How do you draw isotopes?
A: To draw isotopes, represent the nucleus of the atom with the correct number of protons and neutrons. Use the atomic symbol to show the element’s atomic number and mass number.
Q: What is the difference between an isotope and an ion?
A: An isotope is an atom of the same element with different numbers of neutrons, while an ion is an atom that has gained or lost electrons.
Q: How can you use the periodic table to draw isotopes?
A: The periodic table provides information on an element’s atomic number, which corresponds to the number of protons in the nucleus. The atomic mass can also be found on the periodic table, which is the sum of the protons and neutrons in the nucleus.
Gallery
Climate Science Investigations South Florida - Temperature Over Time

Photo Credit by: bing.com / hydrogen deuterium water heavy mass isotopes molar atom oxygen isotope ratio proton science many module gif electrons know metabolic fatigue
The Three Isotopes Of Hydrogen - Buy This Stock Vector And Explore
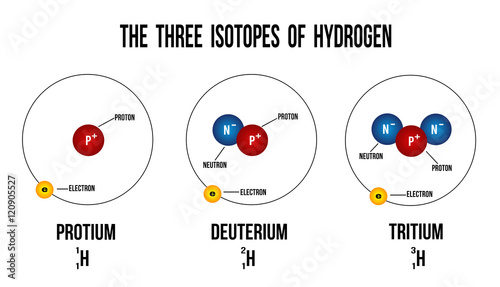
Photo Credit by: bing.com / isotope isotopes hydrogen isotopi wasserstoffs idrogeno isotopen worldatlas neutrons waterstof drie hydrogène elettrone kol atoms diagramma tritium vecteurs
What Are Isotopes, Isobars And Isotones?- Information Palace

Photo Credit by: bing.com / isotopes isotope isobars isotones
Illustrated Glossary Of Organic Chemistry - Isotope

Photo Credit by: bing.com / isotope isotopes glossary proton deuterium
Definisi Isotop Dan Contoh Nya Dalam Ilmu Kimia – Sains Kimia

Photo Credit by: bing.com / isotopes isotop idrogeno isotope isotopi nuclear kimia definisi chemical hydrogen atoms piccaro abundance electron